Evonik Series Filaments
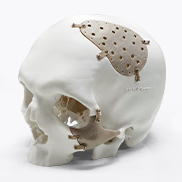
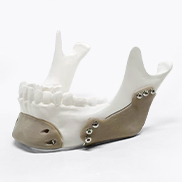
Applicable to customized medical device implant product development and production, etc.
Evonik specializes in providing new solutions in the innovative growth area of additive manufacturing. Its strategic focus is on the development and manufacture of new high-performance materials for mainstream 3D printing technologies. As the world's leading manufacturer of high-performance polymers for additive manufacturing for more than two decades, Evonik offers the healthcare industry a broad portfolio of 3D printed biomaterials for the production of medical devices for short-term or permanent contact with the human body.
Sample test data please add enterprise weibo: wxid_66b59nl99hxu22












































 Home
Home Telephone
Telephone Message
Message







