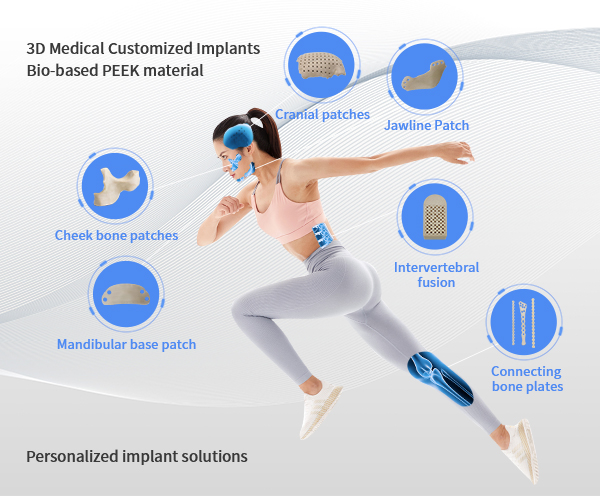
PEEK is used extensively in medical and healthcare solutions. Orthopaedics is a growing area of PEEK application at this stage. More case studies of standardised implants made by additive manufacturing are furthering the wider use of PEEK for medical specialties. In this area, additive manufacturing is becoming an important means of achieving economic viability and production feasibility, providing individually designed as well as tailored implants for specific patients with unique traumas, specialised diseases and birth defects.
A detailed interview with Mr. Popp, Managing Director of German 3D device manufacturer Apium Additive Technologies GmbH, was recently featured in a new Medical Device Developments article in the journal Medical Device Developments. The article focuses on the Apium Bio PEEK 3D printer and highlights how Apium 3D printing technology can be directly and effectively integrated into the hospital's implant manufacturing workflow and build a better future for patients and doctors. Mr.Popp gave expert answers!
Q: How are BioPEEK printers custom manufactured?
A: Bio PEEK printers comply with biocompatibility standards and are specifically designed for medical products, addressing the barriers to the successful incorporation of biocompatible high performance thermoplastics such as PEEK into medical products. At the same time, there must be no contamination when printing PEEK, no alteration to the biocompatibility of the polymer and avoidance of organic and inorganic contaminants. Therefore, all processed parts and end products of the Bio PEEK printer are biocompatible materials. To prevent organic contamination, an integrated filter layer airflow is also provided to blow away dust and bacteria during processing.
Q: What are the features of Bio PEEK printers in terms of innovative manufacturing processes?
A: The higher the molecular weight of PEEK, the higher its mechanical resistivity and viscosity, making it more difficult to print, and therefore requiring greater extrusion force. Our extruders have sufficient pressure and speed to extrude the high molecular weight PEEK material in its molten state out of the nozzle. When printing PEEK we have to ensure efficiency and avoid errors by using coded servo motors, which do not lose step lengths as stepper motors do. There will be no inter-layer movement or drift that could lead to defective parts. Our integrated camera allows the user to see the process as it is happening; the camera can detect if a part is coming off and stop the print to avoid further damage and waste. Once printing is complete, a print log file can be generated to trace the complete printing process.
Q:How do you ensure that the printing system is fully compatible with Evonik's materials?
A:We have been working with Evonik for a long time on the right type of PEEK for medical products. Some PEEK types are easier to flow but have lower mechanical resistivity, and vice versa. Together we have identified types that are extremely suitable in terms of flow and mechanical properties. Although all thermoplastic materials can be used, bio-PEEK printers are made suitable for biocompatible polymers in order to avoid contamination. One can think of bioresorbable materials that dissolve in the body, as well as various products that use thermoplastics, such as valves and gears for dialysis machines.
Q: Which medical device category can products printed by BioPEEK printers reach?
A: Products printed by the BioPEEK printer using Evonik VESTAKEEP® i4 3DF wire fall into Class II. Higher level permanent implants can also be printed, which means that all lower level products can be printed.
Q: How do you support your customers in complying with regulations?
A: We provide the necessary documentation to meet installation and operational qualifications and to support production qualifications. In order to generate medical products we can recommend different software to complete and test medical product designs.
Q: What benefits does the manufacturing process for custom implants bring to clinicians and healthcare professionals?
A: Where clinicians work, the main advantage of 3D printed implants is that they become more involved in the patient-specific design of the implant. Their ideas can be translated directly into designs, and obstacles and manufacturing constraints can be discussed and solutions found together. This leads to faster implant manufacture, reduced surgery time, good fit, little need for adjustment and reduced risk of infection or complications.
Q: How can patients benefit from 3D printing innovations?
A: Each tool has its own suitable use. For bio-PEEK printers, a better application today is patient-specific implants in the craniomaxillofacial region. Production is faster, more cost efficient and easy to integrate into the workflow. Patient-specific implants offer fewer complications for the patient and also facilitate the recovery of our patients.
In recent years, 3D printed PEEK implants have been used in various applications in spine, joints, trauma, oral craniomaxillofacial, neurosurgery and orthopaedics. 3D printing of PEEK personalised orthopaedic implants is a cutting-edge innovative manufacturing method that will become a hot new direction in the orthopaedic implant market and has great market prospects. Shanghai JT Technology has a complete set of data analysis and personalised solutions for 3D printing PEEK implants, feel free to enquire if you need them.






























 Home
Home Telephone
Telephone Message
Message







